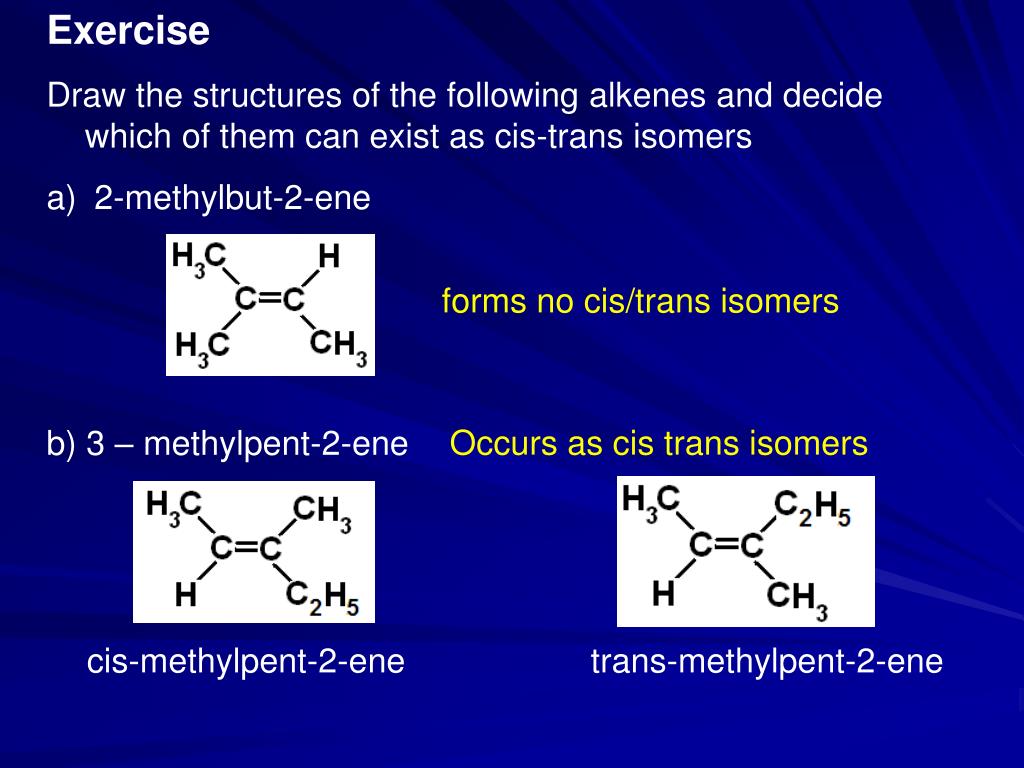
71) Among the following compounds, E / Z isomerism is possible for A) 2methylbut2ene B) 2methylbut1ene C) 3methylpent1ene D) 3methylpent2ene 71) िनÌना ं िकत यौिगकŌ म¤ से िकसम¤ E / Z समावयवता स ं भव है A) 2मेिथल Êयुट2इन B) 2More alkene examples of E/Z isomerism 3methylpent2ene, can be drawn as E/Z isomers using structural and skeletal formula , , methylpent2ene and , Z3methylpent2ene Note the priority order CH 3 CH 2 > CH 3 > H from the CahnIngoldPrelog priority rules (6 CA Use But2ene as an example of E/Z isomerism b Explain why but1ene does not show E/Z isomerism 3 Explain the special case of cistrans isomerism a Use pent2ene as an example of cistrans isomerism b Explain why 3methylpent2ene does not display cis trans isomerism, but does display E/Z isomerism c
Http Ocw Snu Ac Kr Sites Default Files Note 9952 Pdf
2-methylpent-2-ene structure
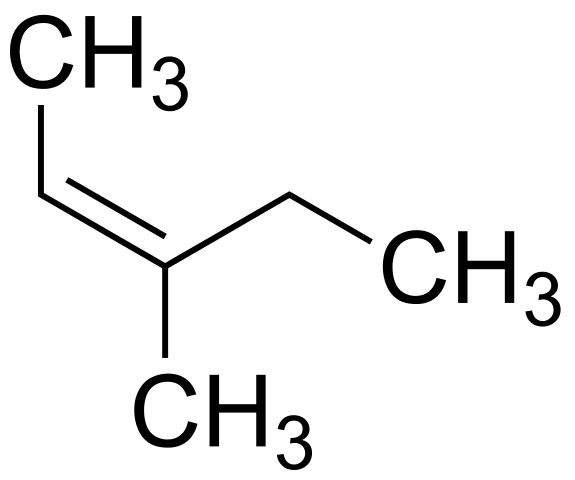
2-methylpent-2-ene structure-(25,3S)2,3dibromopentane (2,4R)2,4dibromopentane (2R,35)2,3dibromopentane (25,3R)2,3dibromopentane The HCH€€€€€€€€€ The alkene (Z)3methylpent2ene reacts with hydrogen bromide as shown below (a)€€€€ (i)€€€€€ Name the major product P (1)




Geometrical Isomerism Cis Trans In Trans 2 Fluoro 3 Methylpent 2 Ene Chemistry Stack Exchange
EZ NOTATION FOR GEOMETRIC ISOMERISM This page explains the EZ system for naming geometric isomers Here is one of the isomers of but2ene The CH 3 group has the higher priority because its carbon atom has an atomic number of 6 compared with an atomic number of 1 for the hydrogen also attached to the carboncarbon double bondThe proper name for this molecule is either trans2fluoro3methylpent2ene because the alkyl groups that form the backbone chain (ie, methyl and ethyl) reside across the double bond from each other, or (Z)2fluoro3methylpent2ene because the highestpriority groups on each side of the double bond are on the same side of the double bond Fluoro is the highestpriority groupYes I was just unsure about it because I thought cistrans isomers were also e/z isomers Well, in a sense they are, but e/z isomers have more than three functional groups Glad you figured it out I think either cistrans or E/Z could be used to describe
(b) Cyclohexene (d) 2, 3dimethylpent2ene ?Which of the follo (a) 3methylpent2ene 2methylpent2ene(a)€€€€ The alkene 3methylpent2ene (CH3CH=C(CH3)CH2CH3) exists as E and Z stereoisomers Draw the structure of Z3methylpent2ene (1) 4 (b)€€€€ Name and outline the mechanism for the formation of 3bromo3methylpentane from this reaction of 3methylpent2ene with hydrogen bromide
2 Decide which of the following alkenes exhibit geometric (EZ) isomerism For those that do, sketch the two stereoisomers to show the isomerism clearly a methylpropene b 2methylpent2ene c 3,4dimethylhex3ene d 1chloropropene e 2ethylpent1ene f 2bromo3chlorobut2eneWhich one of the following is the correct name for answer choices 2bromo3methylpent2ene 2bromo3ethylbut2ene 3bromo2ethylbut2ene 4bromo3methylpent3ene s Question 9 SURVEY212 General formula notes on the various associated unsaturated series of hydrocarbons and the many styles of representing the molecular formula and structure of alkenes The open chain alkenes with one 'ene' group have the general formula C n H 2n (n = 2, 3, 4 etc), they are isomeric with cycloalkanes from C 3 onwards n must be >1 to give a C=C double bond




Which Of The Following Compounds Shows Geometrical Isomerism G I Youtube




Stereoisomerism Wikiwand
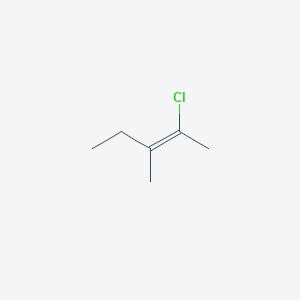
The proper name for this molecule is either trans2fluoro3methylpent2ene because the alkyl groups that form the backbone chain (ie, methyl and ethyl) reside across the double bond from each other, or (Z)2fluoro3methylpent2ene because the highestpriority groups on each side of the double bond are on the same side of the double bond Fluoro is the highestpriority group But1ene is a structural isomer of But2ene but does not show EZ isomerism C C C C H H H H H H H H 1 First determine the priority groups on both sides of the double bond Cl C C Cl H H Cl C C H H Cl N Goalby chemreviseorg Electrophilic Addition Reactions of Alkenes Definition Electrophile an electron pair acceptorGeometric isomerism (also known as cistrans isomerism or EZ isomerism) is a form of stereoisomerism This page explains what stereoisomers are and how you recognise the possibility of geometric isomers in a molecule Further down the page, you will find a link to a second page which describes the EZ notation for naming geometric isomers
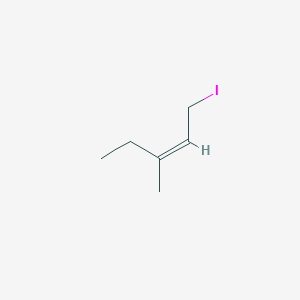



Z 1 Iodo 3 Methylpent 2 Ene C6h11i Pubchem




Among The Following Compounds E Z Isomerism Is Possible For A 2 Methylbut 2 Ene B 2 Methylbut 1 Ene C 3 Methylpent 1 Ene D 3 Methylpent 2 Ene No Links Chemistry States Of Matter Meritnation Com
Click here👆to get an answer to your question ️ 2 Geometrical is a) but1ene 2,3dime Wound Isomerism etrical isomerism is possible in (b) 2methyl but2ene 3dimethyl but2ene (d) 2chloro but2ene the following exhibits geometrical isomerism?According to my own work they do but I just want want to double check 0 EZ isomerism priority rules confusion, please help pls help me im realy stuck chemistry aThe other isomers of pentene are pent2ene represented by the structural formula 2methylbut1ene, represented by the structural formula 3methylbut1ene, represented by the structural formula and 2methylbut2ene, represented by the structural formula where the double lines between the carbon atoms represent a double covalent bond




13 0 Alkenes Exam Q S Flashcards Quizlet
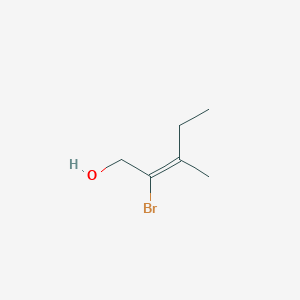



E 2 Brom 3 Methylpent 2 En 1 Ol C6h11bro Pubchem
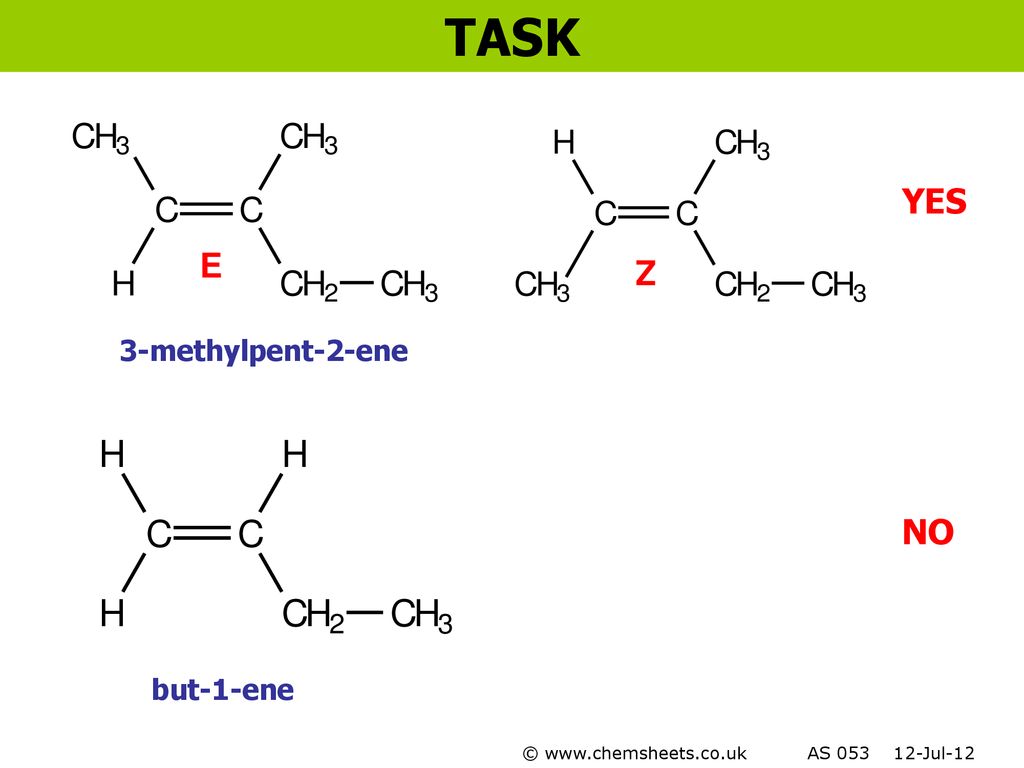
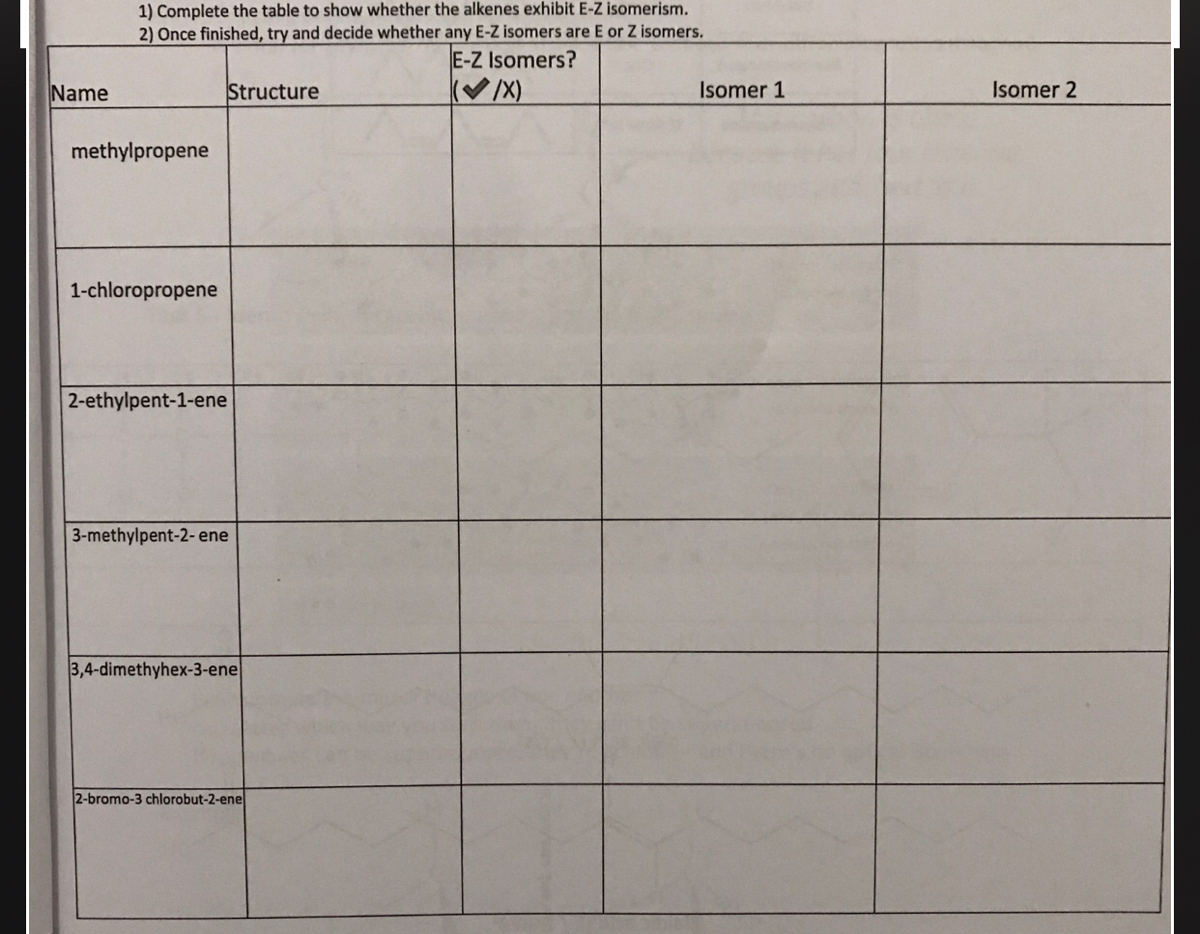
In the E– Z system of nomenclature, the geometry of the isomers is determined by prioritizing the substituents on the two carbons of the double bond The priorities of the substituents are determined by the atomic number with atoms of a higher atomic number having higher priority According to this method 1Stereoisomers to show the isomerism clearly methylpropene 2methylpent2ene 3methylpent2ene 3,4dimethylhex3ene 1chloropropene 2ethylpent1ene but1ene 2bromo3chlorobut2ene CH 3 CH 2 CH 2 CH 3 butane methylpropane H 3 CH 3 eg C 4 H 10 CH 3 CH 2 CH 2 OH propan1ol propan2ol H OH eg C 3 7 OH C H 3 CH 2 C O H propanalGeometric Isomers Practice I For each molecule below, 1) determine whether geometric isomerism is possible 2) name the molecule (use cistrans naming where applicable) a) e) geometric isomerism trans2methylhex3ene geometric isomerism cis4,5,6trichlorohex2ene b) f) geometric isomerism cis4chlorohex2ene geometric isomerism trans8



Ppt Chemistry 3 5 Powerpoint Presentation Free Download Id




Organic Chemistry Alkenes
Bioaccumulation Estimates from Log Kow (BCFWIN v217) Log BCF from regressionbased method = 1670 (BCF = 4679) log Kow used 308 (estimated) Volatilization from Water Henry LC 0359 atmm3/mole (estimated by Bond SAR Method) HalfLife from Model River hours (5626 min) HalfLife from Model Lake 8715 hours (3631 days) Removal Figure 01 EZ nomenclature of 3methylpent2ene In the above image, the E isomer has high priority substituents on the opposite sides of the double bond whereas the Z isomer has those substituents on the same side13methylpent2ene II 2methylbut2ene III 2,3dimethylbut2ene A Only ОВ I And 11 Cland D And Ill Which Of The Following Is The Enantiomer Of (2,3R)2,3dibromopentane?




Which Of The Following Is The Major Product In The Electrophilic Addition Of Hcl To 2 Methylpent 2 Ene Homeworklib
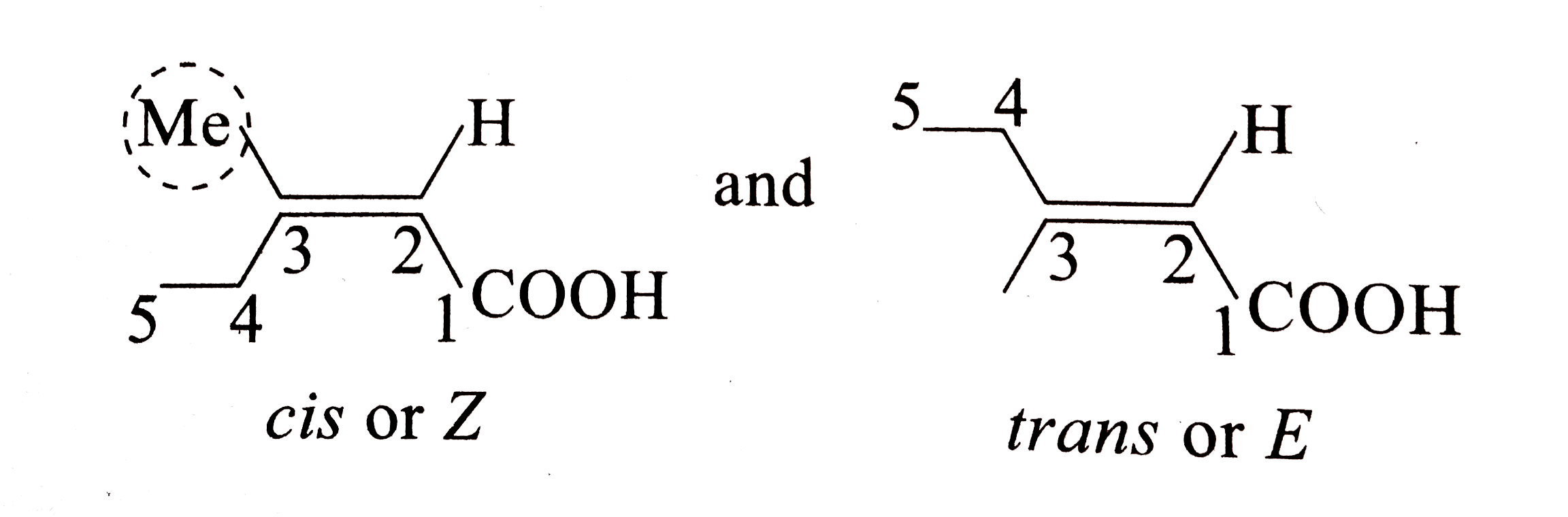



Which Of The Following Compounds Shows Geometrical Isomerism G I A But 2 Ene B 3 Methyl But 2 Enoic Acid C 3 Methyl Pent 2 Enoic Acid D 3 Phenyl Prop 2 Enoic Acid
EZ Isomerism Questions 1 State the meaning of the term stereoisomers 2 Identify the feature of the double bond in but2ene that causes it to form two EZ stereoisomers 3 Draw stuctural formulae of the EZ isomers of the following compounds a) 3methylpent2ene b) but2ene c) 3methylpent2ene d) pent3en2ol e) hex3ene f) pent2ene 4No, 2methyl2pentene does not have cistrans isomers Its formula is (CH3)2C=CH(C2H5) Since one of the doublebonded carbon has two identical groups (methyl groups), this compound cannot exhibit geometrical isomerismBioaccumulation Estimates from Log Kow (BCFWIN v217) Log BCF from regressionbased method = 1709 (BCF = 5123) log Kow used 313 (estimated) Volatilization from Water Henry LC 05 atmm3/mole (estimated by Bond SAR Method) HalfLife from Model River hours (5623 min) HalfLife from Model Lake 8715 hours (3631 days) Removal In




Organic Chemistry Alkenes



Z 2 Chlor 3 Methyl 2 Penten C6h11cl Pubchem
trans2fluoro3methylpent2ene this compound in also known as (Z)2fluoro3methylpent2ene I have given my possible structure in the image but it is wrong, Can anyone explain this ?(E)2chlorobut2ene The E and Z style is more reliable and particularly suited to tri or tetrasubstituted alkenes, and especially when the substituents are not alkyl groups The CahnIngoldPrelog priority rules are used for naming geometric isomers ( eg E or Zalkenes) and other stereoisomers (see later)GEOMETRIC ISOMERISM Z 2bromo2fluorobut2ene EZ nomenclature is based upon the sequence rules developed by Cahn Prelog and Ingold PRIORITY H < CH 3 < CH 2 CH 3 Highest priority groups on opposite side = E Highest priority groups on same side = Z F C C C H 3 C H 3 B r 12 35 9 12 H C C C H 2 C H 3 C H 3 C H 3 12, 12 12 1 12 E 3methylpent2ene



Optical Isomerism Same Molecular Formula But Different Arrangement




Solved Identify The One Which Shows E Z Mechanism A 3 Methylpent 2 Ene 1 Answer Transtutors
Cyclic alkanes In A Level Chemistry, you only need to know about cistrans isomerism due to the presence of a C=C bond What is here?I'm working on an E/Z isomer question (again) and was wondering if 3methylpent2ene had E and Z isomers?I CH_3CH_2CH=CHCH_2CH_3 II (CH_3)_2C = CHCH_3 III CH_3CH_2CH = CHBr IV H_2C = CHCH_2CH(CH_3)_2 A) land II B) I and III C) II and IV D) I II and III The acidcatalyzed dehydration of the alcohol shown below gives a major product which results from a carbocation rearrangement



Z 3 Ethyl 4 Methylpent 2 Ene C8h16 Chemspider



Ib Chemistry On Stereoisomers E Z Cis Trans Geometric Optical And
E/z (cis/trans) isomerism homolytic and heterolytic fission electrophiles and nucleophiles free radicals, electrophiles and nucleophiles classification of reactions i classification of reactions ii test question i 3methylpent2ene yes no The first unnamed isomer is 2methylprop1ene It is a structural isomer of the Ebut2ene and Zbut2ene but does not show EZ isomerism as, despite having a >C=C< with restricted rotation, C1 has two identical groups attached This means that if the bond was broken and we rotated the groups, the product formed would be identical(X) Name Structure Isomer 1 Isomer 2 methylpropene 1chloropropene 2ethylpent1ene 3methylpent2 ene 3,4dimethyhex3ene 2bromo3 chlorobut2ene Fatty acids (265 Q3) Guidance on writing Q3 Why monounsaturated fatty acids have have E/Z isomerism Example of E isomer and how to differentiate to Z Isomer Health issues associated with Z



File Z 3 Methylpent 2 Ene 0 Svg Wikimedia Commons
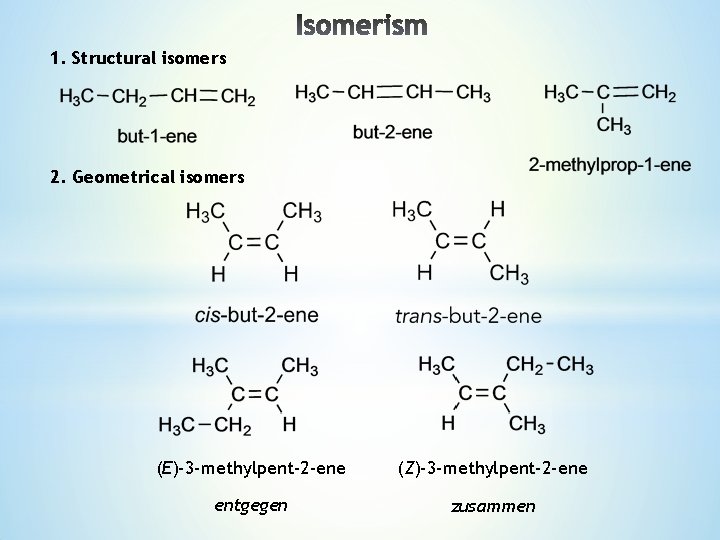



Lecture 4 1 Structural Isomers 2 Geometrical Isomers
The proper name for this molecule is either trans2fluoro3methylpent2ene because the alkyl groups that form the backbone chain (ie, methyl and ethyl) reside across the double bond from each other, or (Z)2fluoro3methylpent2ene because the highestpriority groups on each side of the double bond are on the same side of the double bond Fluoro is the highestpriority groupWhich of the following alkenes exhibit EZ isomerism?2 3methylpent2ene exists as E and Z isomers Explain why these isomers exist and draw the structure of the Z isomer each C atom in C=C has two different groups attached no rotation around C=C (3) 3 The amino acid alanine (also known as 2aminopropanoic acid) is optically active a Draw the structure of alanine (1)



E 3 Ethyl 4 Methylpent 2 Ene



E 2 Chloro 3 Methyl Pent 2 Ene Chemsink
EZ isomerism is one type of this isomerism It applies to alkenes and other organic compounds that contain C=C bonds cyclic alkanes You will be able to identify some of the organic compounds you meet at A Level as cis or trans isomers However,You can see models of the two cistrans isomers of but2eneCistrans isomerism is one type of this isomerism It applies to alkenes and other organic compounds that contain C=C bonds;
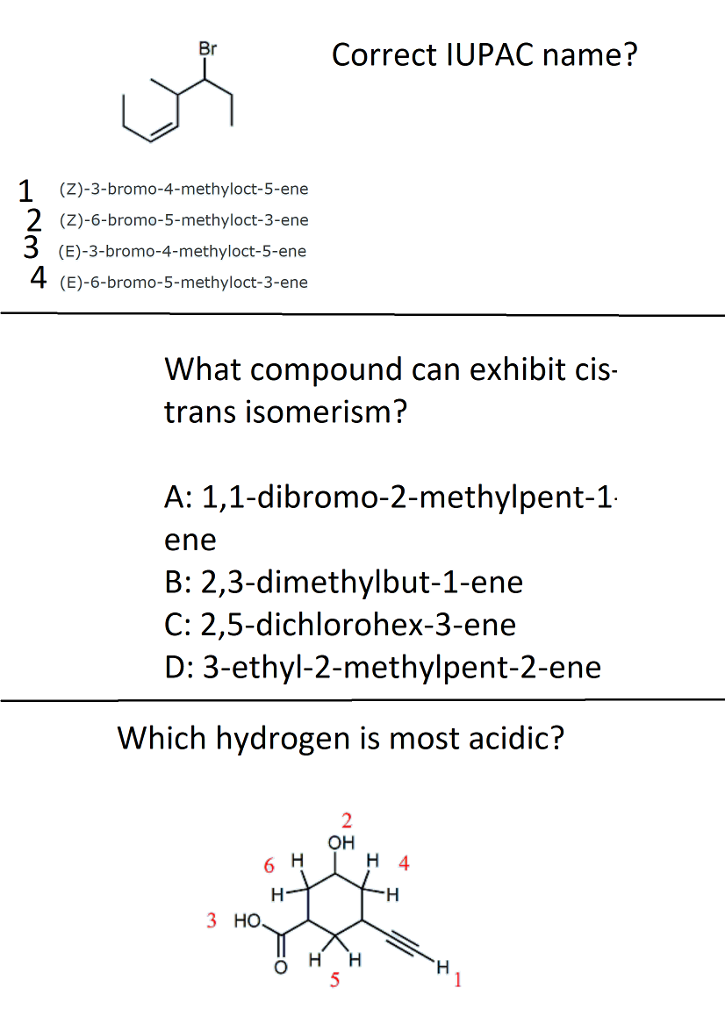



Br Correct Iupac Name Z 3 Bromo 4 Methyloct 5 Ene Chegg Com



Solved Which Of The Following Can Exhibit Geometrical Isomerism Course Hero
2Methyl1pentene 2methyl2pentene C12H24 CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and moreHexene is an alkene with a molecular formula C 6 H 12The prefix "hex" is derived from the fact that there are 6 carbon atoms in the molecule, while the "ene" suffix denotes that there is an alkene present—two carbon atoms are connected via a double bondThere are several isomers of hexene, depending on the position and geometry of the double bond in the chainThere are two forms of stereoisomerism – E/Z isomerism and optical isomerism E/Z Isomerism The C=C bond is central to E/Z isomerism Compounds have different atoms or groups 2chloro,4methylpent2ene (Z) 2chloro,4methylpent2ene 4 Optical isomerism As the name suggests, optical isomers influence light They rotate the plane



1



Issr Edu Kh Science As and ib worksheets 10 Organic Chemistry Quick check Chemsheets 1122 Qc Isomerism Ans Pdf
Question Which Of The Following Alkenes Exhibit EZ Isomerism?(Z)3Methyl2penten1ol C6H12O CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more Now there are two possible isomers, because the = is inflexible The Zisomer where the stuff left of the = is at the same side of the C = C as the stuff on the right, so both down or both up (from German Zusammen=together) The Eisomer where they are on opposite sides (from Entgegen=opposite) Note They are also called cis (Z) and trans (E)




Structure Of The Molecule Of Download Scientific Diagram



Aqa As Jun 16 Paper 2 Q7 With Explained Solutions




The Correct Iupac Name Of The Following Compound Is




Difference Between E And Z Isomers Compare The Difference Between Similar Terms
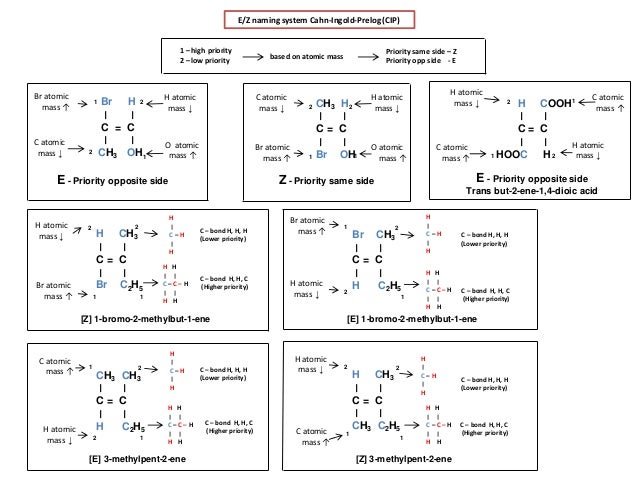



Ib Chemistry On Stereoisomers E Z Cis Trans Geometric Optical And




11 Which One Would Be E Isomer A B Br Oh 12 Chegg Com




Geometrical Isomerism Cis Trans In Trans 2 Fluoro 3 Methylpent 2 Ene Chemistry Stack Exchange




3 Methyl Pent 2 Ene On Reaction With Hbr In Presence Of Peroxide Forms An Addition Product The Number Of Possible Stereoisomers For The Products



Organic Nomenclature Ii



Cis 3 Methylpent 2 Ene




Name All Of The Branched Chain Alkene Isomers C 6h 12 That Contain A Single Methyl Branch And That Have E Z Isomers Study Com
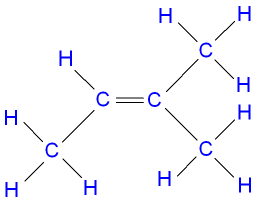



Gcse Chemistry What Are The Isomers Of Pentene Pent 1 Ene Pent 2 Ene 2 Methylbut 1 Ene 2 Methylbut 2 Ene 3 Methylbut 1 Ene Gcse Science




Chemsheets As006 Electron Arrangement Ppt Video Online Download



2e 3 Methylpent 2 Ene Get Quote




Help Mcat




E Z Isomerism Cahn Inglod Prelog Priority Rules Examples Explained Drawings Diagrams Difference In Physical Chemical Properties Cis Trans Isomers For Given Formula A Level Gce As Organic Chemistry Revision Notes



Z 3 Methyl 2 Pentene C6h12 Chemspider




Ch3 7 5 Name C Ch2ch3 A Trans Pent 2 Ene B Chegg Com




E Z Isomerism Cahn Inglod Prelog Priority Rules Examples Explained Drawings Diagrams Difference In Physical Chemical Properties Cis Trans Isomers For Given Formula A Level Gce As Organic Chemistry Revision Notes




3 Methyl 3 Penten 2 One Wikipedia




E Z Isomerism Card Match Teaching Resources
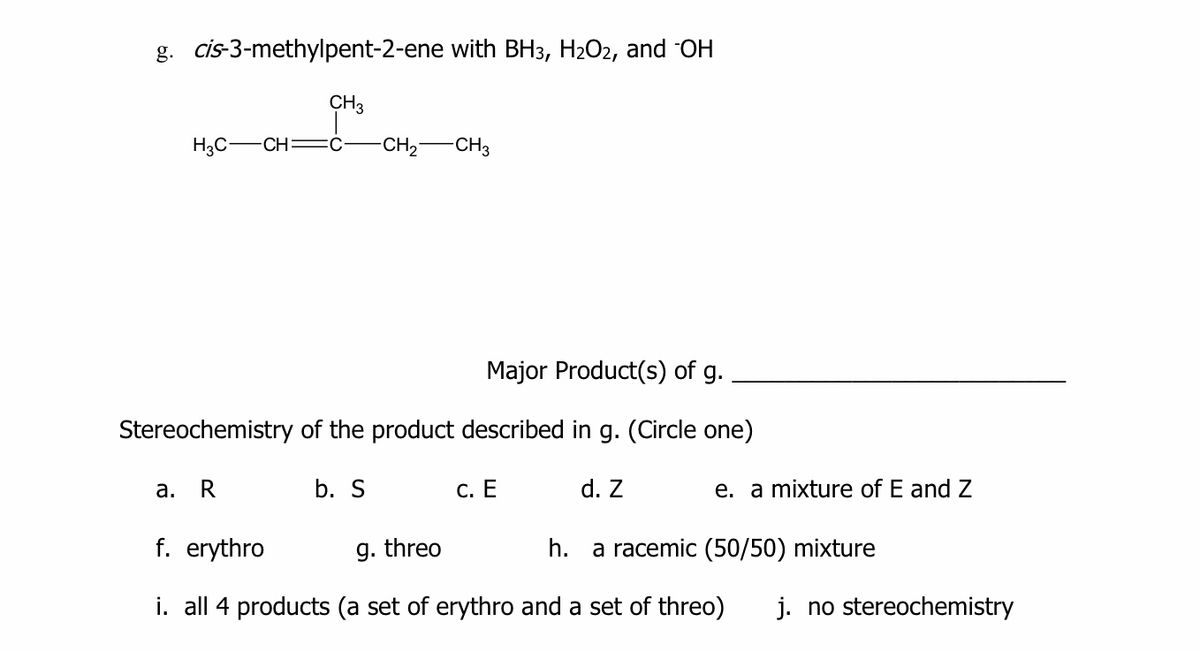



Answered G Cis 3 Methylpent 2 Ene With Bh3 Bartleby



Does 3 Methyl 2 Pentene Have Cis Trans Isomers Quora



How Many Different Acyclic Isomers Of C6h12 On Hydrogenation Class 12 Chemistry Cbse



Z 3 Methylpent 2 Ene Chemsink



Which Of The Following Compounds Are Capable Of Ciis And Trans Isomerism Why 4 Methylpent 2 Ene 3 Methylpent 1 Ene 4 Chlorohex 2 Ene Quora



Molecules Free Full Text Synthesis And Use Of Stable Isotope Enriched Retinals In The Field Of Vitamin A Html




Draw E And Z Isomers Of Pent 2 Ene Brainly In
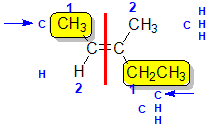



E And Z Alkenes




Write The Structural Formula And Name The Product S For A 4 Methyl Pent 2 Ene With Potassium Permanganate Solution B Homeworklib



2 Pentene 3 Methyl E



Echa Europa Eu Substance Information Substanceinfo 100 010 561



When Is The Cis Trans System Not Effective For Naming Geometric Isomers Socratic



2z 3 Methylpent 2 Ene Get Quote




File Z 3 Methylpent 2 Ene 0 Svg Wikimedia Commons




3 Methyl 2 Pentene Cis And Trans Mixture 99 0 Tci America Fisher Scientific




Which Of The Following Exhibits Geometrical Isomerism A 3 Methylpent 2 Ene B Cyclohexene C 2 Methylpent 2 Ene D 2 3 Dimethylpent 2 Ene Chemistry Organic Chemistry Some Basic Principles And Techniques Meritnation Com




Among The Following Compouds E Z Isomerism Is Possible For A 2 Methylbut 2 Ene B




2e 2 Chloro 3 Methylpent 2 Ene Structure C6h11cl Over 100 Million Chemical Compounds Mol Instincts



Http Themathsandsciencetutor Co Uk Wordpress Wp Content Uploads 02 Alevelchemistry Mechanisms Pdf



1



Chemsheets As006 Electron Arrangement Ppt Download
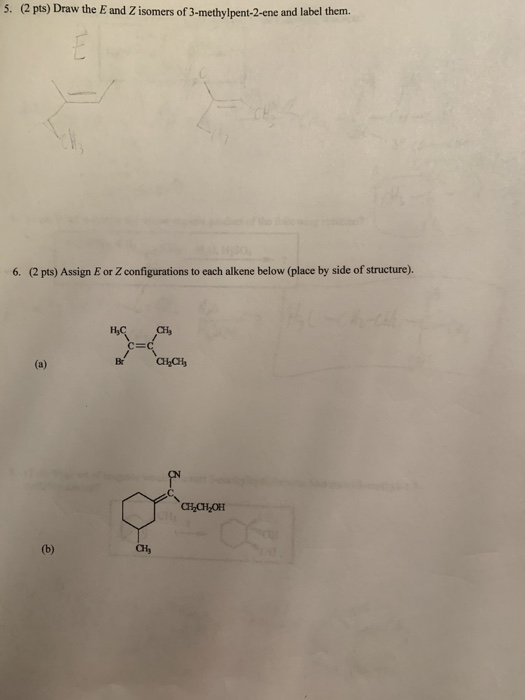



1 Draw The E And Z Isomers Of 3 Methylpent 2 Ene And Chegg Com



Orgchemboulder Com Examarchives 51hyf12 51hyf12ex2 Pdf




E Z Isomerism Cahn Inglod Prelog Priority Rules Examples Explained Drawings Diagrams Difference In Physical Chemical Properties Cis Trans Isomers For Given Formula A Level Gce As Organic Chemistry Revision Notes



Http Ocw Snu Ac Kr Sites Default Files Note 9952 Pdf




Cis But 2 Ene An Overview Sciencedirect Topics
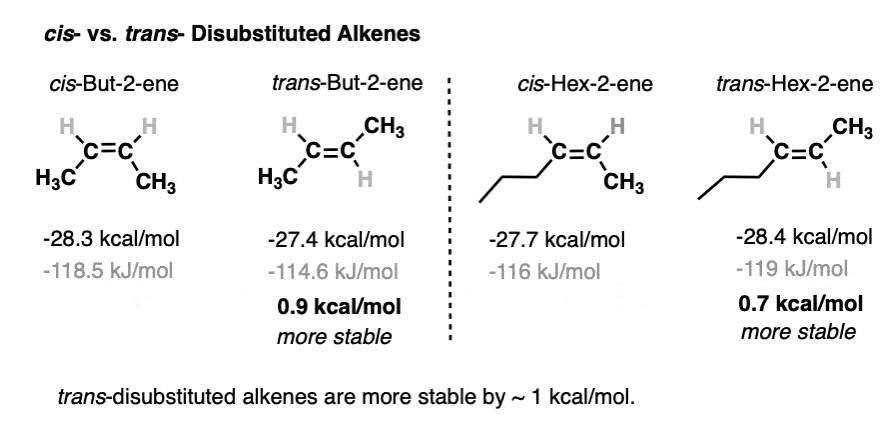



Alkene Stability Increases With Substitution Master Organic Chemistry




13 0 Alkenes Exam Q S Flashcards Quizlet



1



Answered E Z Isomers Name Structure X Isomer Bartleby




File Z 3 Methylpent 2 Ene 0 Svg Wikimedia Commons



How Many Different Alkenes Can Be Hydrogenated To Form 3 Methylpentane Socratic




Alkene Nomenclature Video Khan Academy




Draw The Structure S Of The Alkene S With The Molecular Formula C6h12 That Have A Single Methyl Homeworklib



2e 3 Methyl 2 Pentene C6h12 Chemspider



1



Solved B Draw And Clearly Label Z And E Isomers For Any Of The 10 Compounds That Have Geometric Isomers Course Hero
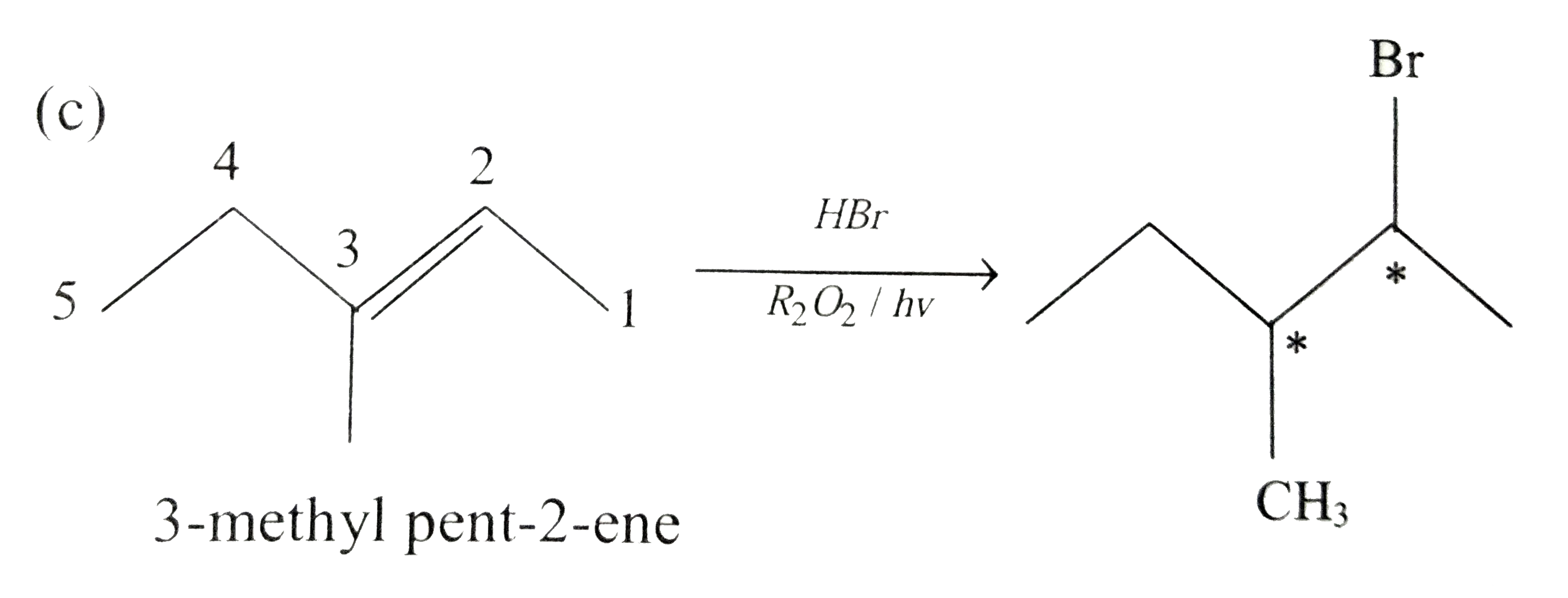



3 Menthyl Pent 2 Ene On Reaction With Hbr In Presence Of Peroxide Forms An Addition Product The Number Of Possible Stereoisomers For The Product Is



Z 3 Ethyl 4 Methylpent 2 Ene




2e 2 Chloro 3 Methylpent 2 Ene Structure C6h11cl Over 100 Million Chemical Compounds Mol Instincts




Cis But 2 Ene An Overview Sciencedirect Topics
-2-bromo-3-methylbutane_small.png)



Organic Chemistry Alkenes




Do Now What Is The Definition Of A Structural Isomer Ppt Download



Ass 1aa3 Org
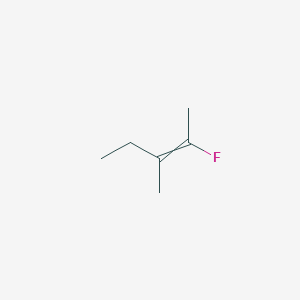



2 Fluoro 3 Methylpent 2 Ene C6h11f Pubchem




E Z Isomerism Cahn Inglod Prelog Priority Rules Examples Explained Drawings Diagrams Difference In Physical Chemical Properties Cis Trans Isomers For Given Formula A Level Gce As Organic Chemistry Revision Notes
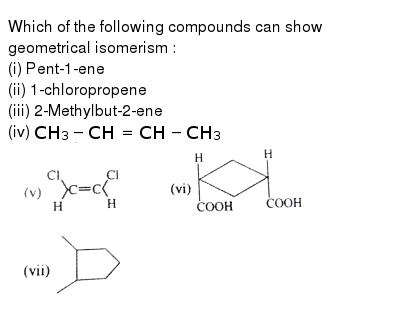



Among The Following Compouds E Z Isomerism Is Possible For A 2 Me




Which Of The Following Alkenes Exhibit E Z Isomerism Chegg Com



2 Methylpent 2 Ene Get Quote




Rank The Alkenes Below From Least To Most Stable A 2 3 Dimethylpent 2 Ene B 3 Ethylpent 2 Ene C 2 Methylhex 1 Ene D Cis Hept 2 Ene E Trans Hept 2 Ene F Hept 1 Ene Study Com



Echa Europa Eu Substance Information Substanceinfo 100 009 515




Stereoisomerism Wikipedia




Among The Following Compounds Eiz Isomerism Is Possible For
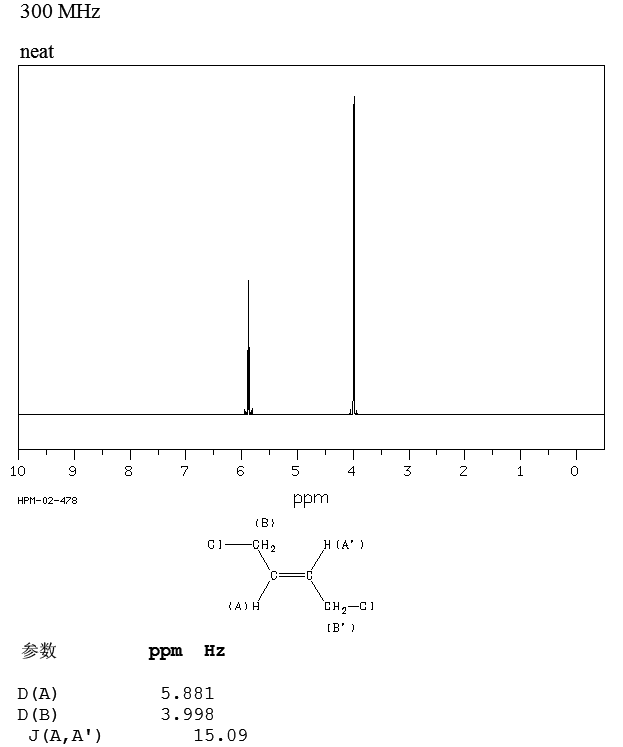



How Can You Distinguish E And Z Isomers By Nmr Socratic



Z 4 Methyl 2 Pentene C6h12 Chemspider




Types Of Isomerism Structural Isomerism Stereoisomerism Geometrical Isomerism Optical Isomerism Simple Isomerism Before You Start It Would Be Ppt Download
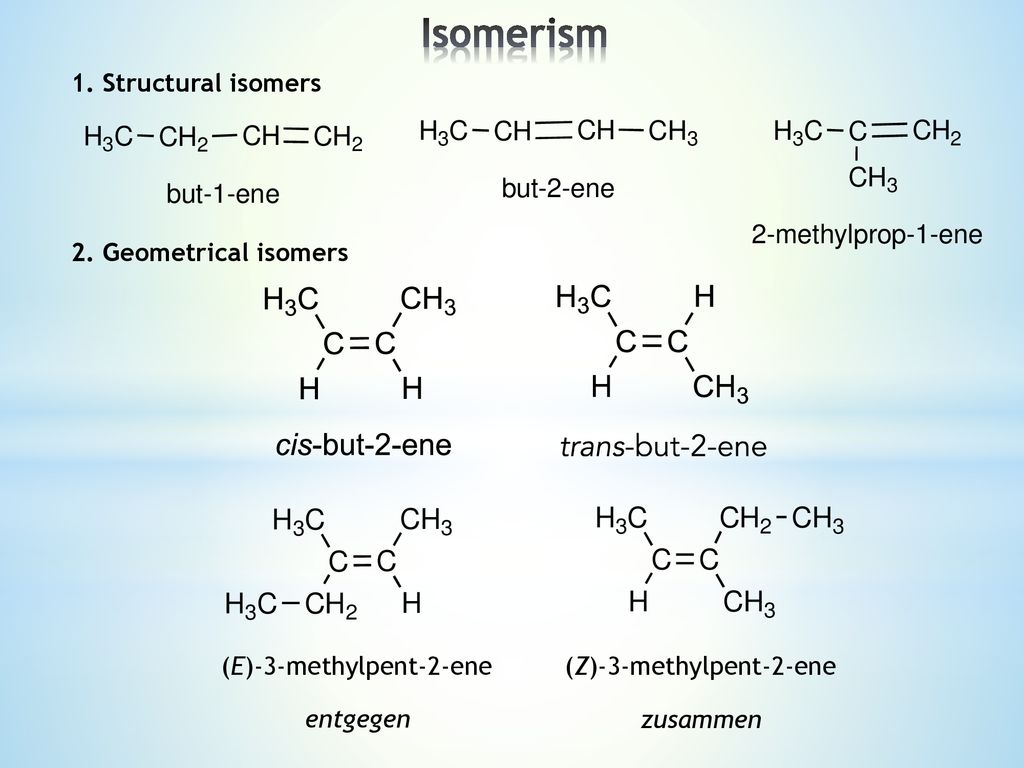



Alkenes Lecture Ppt Download
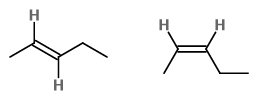



What Are The E And Z Isomers Of Pent 2 Ene Socratic
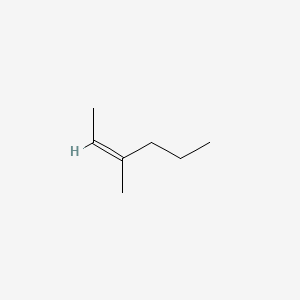



Z 3 Methyl 2 Hexene C7h14 Pubchem



0 件のコメント:
コメントを投稿